Pharma Equity Group (“PEG” or “the Company”) announced on Friday, April 5th, that the Company’s subsidiary Reponex Pharmaceuticals A/S (“Reponex”) has received positive final results from the Phase II clinical proof-of-concept trial of the drug candidate RNX-051.
The Phase II trial, also referred to as the MEFO trial, concerns the treatment of patients with right-sided colon cancer and right-sided colon polyps/adenomas (precursors of cancer) with the Company’s drug candidate RNX-051. The trial consisted of two arms: the first in patients with adenomas (the “adenoma arm”) and second in patients with cancers in the right side of the bowel (the “cancer arm”). In the adenoma arm, the main goal of the study, to demonstrate an impact on the bacterial biomass, was reached, with a massive reduction in the biofilm of the bowel lining (more than 30-fold reduction). In the cancer arm, for patients with a high content of bacterial biofilm, there was a statistically significant reduction of biofilm in the tumor periphery.
Reponex’s management concludes that its patented medicinal product RNX-051 appears to be highly effective for its intended purpose. Just a single local application drastically reduces tumor-associated biofilm and can even totally eliminate the cancer-promoting Fusobacterium nucleatum in the tumor one week after the treatment.
Analyst Group’s view
“The positive results obtained from the Phase II study are a further demonstration from Reponex that the clinical development is progressing according to plan. The recently strengthened cash position following the convertible loans provides the Company with additional room to maneuver. Coupled with clinical progression, it de-risks the investment case and reinforces PEG’s negotiation power in discussions with potential licensing partners.
During 2020, approximately 12.7% of new cancer diagnoses and 12.4% of cancer-related deaths were attributed to colorectal cancer in EU-27 countries, making it the second most prevalent cancer, following breast cancer, and the second leading cause of cancer-related mortality after lung cancer.1 Hence, the demand for an effective and localized treatment solution is critical.
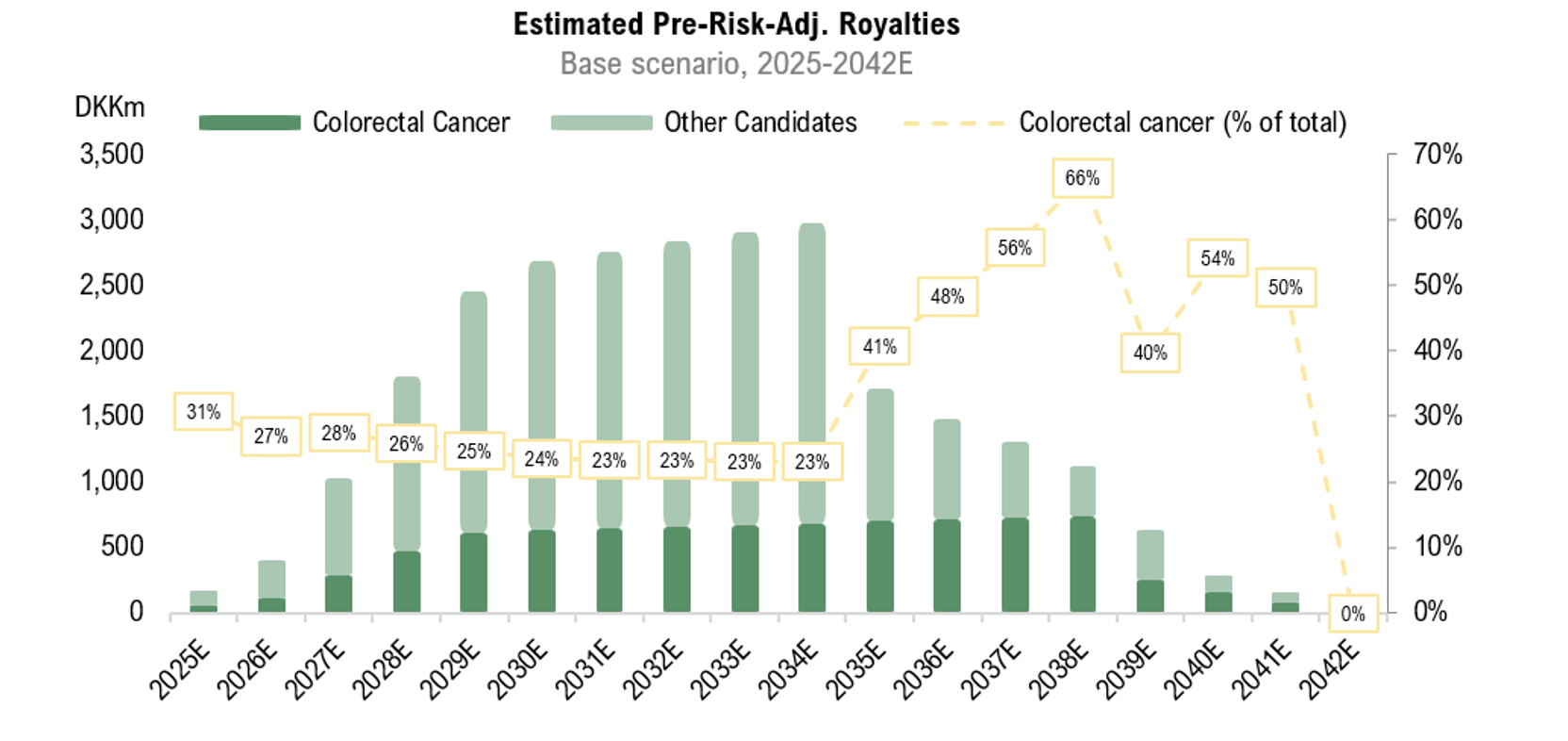
Analyst Group estimates that the potential royalties from RNX-051 will constitute a significant portion of the total pre-risk-adjusted royalties, making it a key candidate for future potential cash flow streams. The figure below illustrates Analyst Group’s estimates for colorectal cancer (RNX-051).”
Analyst Group’s View of Pharma Equity Group:
Pharma Equity Group (“PEG” or “the Company”), through the Company’s subsidiary, Reponex, employs a drug repositioning strategy, which involves finding new uses for active substances used in previous recognized treatments, thus allowing the Company to circumvent phase I trials. PEG has a pipeline of six candidates in Phase II, targeting therapeutic areas such as Peritonitis, Chronic Wounds, IBD, and Colorectal Cancer, where there is currently no adequate treatment. The business strategy involves out-licensing the programs after Phase II to a pharma company capable of bringing the drugs to the market. PEG’s strategy enables a capital-light and highly scalable business model, offering a shorter route to market with equivalent upside potential, yet mitigating the typical risks associated with the pharmaceutical industry. Based on an rNPV-model, a potential present value per share of DKK 1.4 is derived in a Base scenario.
You can access our initial analysis of Pharma Equity Group here, and also watch a recent interview with the CEO, Thomas Kaas Selsø here.
1https://ecis.jrc.ec.europa.eu/pdf/factsheets/Colorectal_cancer_en-Mar_2021.pdf


