Pharma Equity Group A/S (“PEG” or “the Company”), listed on the Nasdaq Copenhagen Stock Exchange, places a strong emphasis on its subsidiary, Reponex Pharmaceuticals A/S (“Reponex”). Through the Company’s repositioning strategy, Reponex finds new uses for active substances that are being used in other treatments. Currently, Reponex has a pipeline of six product candidates in Phase II, targeting therapeutic areas such as Peritonitis, Chronic Wounds, IBD (Crohn’s Disease and Pouchitis), and Colorectal Cancer. PEG’s strategy is to out-license the clinical programs after the Phase II trial to a pharmaceutical company capable of bringing the drugs to market.
Press releases
Advanced Discussions with Prospective Licensing Partners
Pharma Equity Group (“PEG” or “the Company”) has recently refined the Company’s execution strategy, prioritizing resources toward three key candidates, RNX-051, RNX-011, and RNX-041, where the Company identifies the shortest path to market and strong interest from potential licensing partners. Discussions with prospective partners have advanced, particularly for RNX-051, with negotiations intensifying in Q1-25 and the Company anticipating formalizing agreements in H2-25. Supported by a solid financial foundation following the Q4-24 directed share issue, a cost-efficient operational model, and expected revenues in H2-25, PEG is estimated to be financed until Q2-26. With a newly appointed executive team bringing a proven track record in funding, strategic execution, and clinical acceleration, PEG is well-positioned to deliver on the Company’s new strategy. Analyst Group has made slight adjustments to the estimates for 2025-2027, resulting in a potential present market value of DKK 959m, corresponding to DKK 0.8 (0.8) per share in a Base scenario.
- Ongoing Discussions with Potential Licensing Partners
Interest from potential licensing partners has gained momentum in Q1-25 with PEG advancing discussions particularly regarding RNX-051, the Company’s candidate targeting Colon Adenomas and Colon Cancer. PEG expects to finalize agreements in H2-25, as reflected in the Company’s DKK 11m revenue guidance for 2025, primarily driven by upfront payments. Simultaneously, PEG’s focus on cost efficiency is expected to narrow the pre-tax loss to DKK 4–7m, which would mark a notable improvement from 2024. Enhanced financial flexibility strengthens PEG’s ability to capitalize on licensing opportuneities, which Analyst Group identifies as a pivotal catalyst for 2025.
- Financial Flexibility Supports Accelerated Development
During Q4-24, PEG strengthened the Company’s financial position through a directed share issue, raising DKK 51.1m in gross proceeds, including DKK 12.6m from convertible debt conversion. This non-cash transaction resulted in a net cash inflow of DKK 38.5m, with DKK 25.8m allocated to debt reduction, leaving DKK 12.7m in net proceeds. The shares were issued at DKK 0.25, a 19% premium, reflecting strong investor confidence in PEG’s prospects. In Q1-25, PEG secured additional financial headroom through loans and commitments totaling approx. DKK 13m, providing an estimated 12-month runway. Further funding discussions, primarily regarding convertible loans, are ongoing with both existing and new investors.
- Key Value Drivers Emerging in 2025
In light of the Q4 report, we have lowered our 2025-2027 revenue estimates. However, with a more streamlined cost structure expected, we anticipate an improved pre-tax loss (EBT) in the short term. Analyst Group reiterates the motivated potential present value of DKK 0.8 (0.8) per share in a Base scenario, as we see 2025 as a pivotal year for PEG, with substantial licensing triggers yet to be fully reflected in the Company’s valuation.
7
Value drives
1
Historical profitability
7
Management & Board of Directors
8
Risk profile
All analyses of companies from 2020 onwards are rated based on a new rating system - Value Driver, Historical Profitability and Management & Board ranges from 1 to 10, where 10 is the highest rating. The risk profile ranges from 1 to 10, where 10 is to be considered the highest risk. Stock analyses of companies published before 2020 have been rated based on a different model.
Strengthened Balance Sheet Facilitates Continued Development
Following the end of Q3-24, Pharma Equity Group (“PEG” or “the Company”) secured additional funding through a directed share issue, with a significant portion allocated to debt reduction. This strengthens the Company’s financial position, providing increased flexibility to advance the development of PEG’s drug candidates while enhancing the Company’s negotiating leverage with potential licensing partners. Furthermore, PEG recently obtained patent protection in Japan, a key future market, for the treatment of colorectal cancer with RNX-051, valid until 2039. Following a reassessment of market conditions, an updated royalty rate has been applied, resulting in a potential present market value of DKK 950m, corresponding to DKK 0.8 (1.2) per share in a Base scenario.
- Successful Capital Increase Creates Financial Flexibility
In October, PEG successfully completed a directed share issue, issuing 204,592,776 new shares at a subscription price of DKK 0.25 per share, reflecting a premium of approx. 19% compared to the prior trading day’s closing price. The gross proceeds amounted to DKK 51.1m, which included the conversion of DKK 12.6m in convertible debt. Excluding the non-cash component from the debt conversion, the cash proceeds totaled DKK 38.5m, of which DKK 25.8m was allocated to the reduction of financial debt, resulting in net cash proceeds of DKK 12.7m. This capital raise has materially strengthened the Company’s financial position, improving the balance sheet and overall capital structure, thereby enhancing financial flexibility for continued clinical development and negotiations with potential licensing partners.
- Solid Cost Control
During Q3-24, the Company’s operating expenses totaled approx. DKK 5.2m (5.7), reflecting an 8% Y-Y decrease and a 5% Q-Q increase. A detailed analysis of OPEX shows that R&D expenses declined by 29% Y-Y and 3% Q-Q, while administrative expenses rose by 8% Y-Y and 10% Q-Q. The modest sequential increase in the cost base is a natural step as PEG advances its development efforts, taking strides toward securing lucrative licensing agreements. Analyst Group considers PEG’s cost management to be solid, as reflected in the maintained full-year 2024 guidance, which projects EBT in the range of approx. DKK -24m to -29m (excluding potential gains or losses related to the Portinho receivable).
- Revised Valuation Range
Following a reassessment of market conditions and comparable licensing agreements, adjusted royalty rates has been applied, resulting in a potential present market value of DKK 950m, equivalent to DKK 0.8 (1.2) per share. For a detailed explanation of the revised royalty rate, please see page 4. Analyst Group remains of the opinion that the substantial potential of PEG’s drug candidates is not fully reflected in the current valuation, presenting an attractive risk/reward profile. We believe that the low liquidity of the share is one of the key factors contributing to the compressed share price.
6
Value drives
1
Historical profitability
7
Management & Board of Directors
8
Risk profile
All analyses of companies from 2020 onwards are rated based on a new rating system - Value Driver, Historical Profitability and Management & Board ranges from 1 to 10, where 10 is the highest rating. The risk profile ranges from 1 to 10, where 10 is to be considered the highest risk. Stock analyses of companies published before 2020 have been rated based on a different model.
Exploring Opportunities for a Capital Increase
Pharma Equity Group (“PEG” or “the Company”) presented a Q2-report characterized by further advancements in the clinical development (RNX-051), effective cost management, and an intensified focus on securing additional capital. The Company is evaluating options for a capital increase, which is essential for supporting further clinical advancements and for having the financial capacity to explore potential licensing agreements. In light of facing financial pressures, PEG demonstrated robust cost control, evidenced by a 1% increase in total operating expenses Y-Y and a -27% decrease Q-Q. Looking ahead, Analyst Group will monitor the continued clinical progression, the financial position, the EMA’s decision regarding orphan drug designation for RNX-041, the receivable from Portinho S.A., and potential discussions with licensing partners concerning PEG’s strong portfolio of product candidates in Phase II. Analyst Group has made minor adjustments to the discount rate and forecasts, resulting in a revised potential present value of DKK 1.2 (1.4) per share in a Base scenario.
- Improved Cost Control Q-Q
During the second quarter, PEG reported operating costs of approx. DKK 5m, up from DKK 4.9m in Q2-23, reflecting a 1% increase Y-Y and a -27% reduction Q-Q. A detailed breakdown of the cost base reveals a decrease in R&D-expenditures of -27% Y-Y and -28% Q-Q, while administrative costs increased by 30% Y-Y but decreased sequentially by -26% compared to Q1-24. PEG upholds the Company’s guidance for FY2024, with expected EBT in the range of DKK -24 to -29 million, excluding any potential gains or losses related to the Portinho receivable. Analyst Group views the improved cost control Q-Q as crucial given the current liquidity position. However, we anticipate that increased investments in R&D will be necessary in the coming years to achieve further clinical advancements going forward.
- Legal Actions to Redeem Receivable from Portinho S.A.
PEG filed a summons with the Maritime and Commercial High Court against Portinho S.A. during Q2-24 to recover the receivable. As of the end of June, the receivable amounted to EUR 11.0m, including agreed interest, which corresponds to DKK 82.1m. Although Analyst Group has not factored this receivable into PEG’s valuation, it could be crucial for sustaining the Company financially and providing additional upside to the valuation if successfully recovered.
- Revised Valuation Range
Following minor adjustments to the discount rate (WACC) to account for increased financial risk, as well as subtle revisions to the long-term forecast, a potential present market value of DKK 1,243m is derived using an rNPV model, equivalent to DKK 1.2 (1.4) per share. Analyst Group maintains that the substantial potential in PEG’s drug candidates is not currently reflected in the Company’s valuation.
6
Value drives
1
Historical profitability
7
Management & Board of Directors
8
Risk profile
All analyses of companies from 2020 onwards are rated based on a new rating system - Value Driver, Historical Profitability and Management & Board ranges from 1 to 10, where 10 is the highest rating. The risk profile ranges from 1 to 10, where 10 is to be considered the highest risk. Stock analyses of companies published before 2020 have been rated based on a different model.
Clinical Development Progression and Strengthened IP-Portfolio
Pharma Equity Group (“PEG” or “the Company”) presented a Q4-report marked by advancements in the clinical development, the addition of two well-experienced board members, and a bolstered IP portfolio. As PEG’s broad Phase II-pipeline progresses further towards potential licensing agreements, the cost base and burn rate are on the rise, as evidenced by the R&D and administrative costs, marking a 26% and 15% increase Q-Q, respectively. PEG has taken critical measures to reinforce the balance sheet and to ensure a solid financial position going into 2024. These measures include the utilization of convertible loans and the securing of a new credit facility after the end of Q4-23. Analyst Group derives a potential present value of DKK 1,448, equivalent to DKK 1.4 (1.4) per share in a Base scenario.
- Clinical Progression Remains on Track
During Q4-23, the Company unveiled encouraging preliminary findings from the Phase II clinical trial of the drug candidate RNX-051, successfully achieving the trial’s primary endpoints. The comprehensive analysis of the study’s outcomes is anticipated to be disclosed in early 2024, marking a short-term value driver.
- Strengthened IP-Portfolio
Apart from clinical progression, protecting the IP-rights is a cornerstone in the pharmaceutical industry. During the quarter, PEG obtained a granted patent in the US for a method of treatment using its topical wound-healing composition, and following the end of Q4-23, the Company was granted EU patents for drug candidates RNX-051 and RNX-022. Both the US and the EU represent key markets for PEG, and Analyst Group considers these milestones pivotal in the Company’s IP-strategy. A reinforced IP-portfolio not only offers legal protection for the pipeline candidates but also serves as substantial assets during negotiations with potential licensing partners.
- Enhanced Financial Position
During Q4-23 and the beginning of 2024, PEG successfully issued convertible loans totaling DKK 16m and secured a new credit facility, expanding the available credit line to DKK 12.6m. The cash balance at the end of Q4-23 amounted to DKK 4.2m, and with an estimated monthly burn rate of DKK -2.0m, reflecting a period of increased R&D and administrative costs, Analyst Group estimates that PEG will be adequately financed throughout 2024, all else being equal. As PEG relies on external financing until potential licensing agreements materialize, the enhanced financial position is vital.
- Valuation Range Remains Intact
After making slight adjustments to the estimated cost base, Analyst Group maintains the opinion that the vast potential in PEG’s drug candidates is not reflected in today’s valuation. A potential present market value of DKK 1,448m is derived through a rNPV-model, equivalent to DKK 1.4 (1.4) per share.
6
Value drives
1
Historical profitability
7
Management & Board of Directors
7
Risk profile
All analyses of companies from 2020 onwards are rated based on a new rating system - Value Driver, Historical Profitability and Management & Board ranges from 1 to 10, where 10 is the highest rating. The risk profile ranges from 1 to 10, where 10 is to be considered the highest risk. Stock analyses of companies published before 2020 have been rated based on a different model.
Analyst Comments
Comment on Pharma Equity Group’s New Execution Strategy
2024-12-13
Pharma Equity Group (“PEG” or “the Company”) announced on Friday, December the 13th, the board of directors’ decision on a new execution strategy and prioritization of clinical areas regarding the Company’s subsidiary Reponex Pharmaceuticals A/S (“Reponex”).
PEG conducts continuous evaluation of the clinical pipeline in regards to Reponex based on several fundamental commercial criteria, including medical need, patient recruitment, regulatory requirements, likelihood of success, and requirements for both human and monetary capital. Based on this evaluation, Reponex has prioritized the following development programs going forward:
- RNX-051 for Colon Adenomas and Colon Cancer
- RNX-011 for the Treatment of Peritonitis
- RNX-041 for the Treatment of IBD (Pouchitis)
The abovementioned drug candidates have demonstrated relevant, informative, and clinical data, with patent protection secured in the most critical geographical regions for the Company.
Development of RNX-051 and RNX-011
In recent months, PEG and the Company’s clinical partners have allocated significant resources to finalize study protocols in preparation for the submission of Phase 2 clinical trial applications to regulatory authorities. The trial applications for RNX-011 and RNX-051 are expected to be submitted at the beginning and end of Q1-25, respectively. During Q1-25, Reponex will implement laboratory models using blood samples from patients with peritonitis to confirm the disease mechanisms affected by RNX-011. These results will help to determine the correct dosage of the drug and generate an ongoing data package. This initiative will not only support clinical studies but also enable the individualization of treatment for patients with peritonitis.
Development of RNX-041
Regarding RNX-041, the drug candidate is actively included in Part 2 of the ongoing Phase 2 proof-of-concept clinical study for the treatment of pouchitis. The studies are conducted as investigator-initiated trials (IITs), designed in compliance with FDA and EMA guidelines, as well as anticipated requirements from future industrial licensing partners. Furthermore, the study design enables continuous analysis and timely data release.
Recent activities
The Company is also working to establish strategic partnerships, a process that will continue throughout the completion of the abovementioned studies. Additionally, Reponex has unblinded the proof-of-concept study with RNX-021 for the treatment of chronic venous leg ulcers. As anticipated, data from the study have provided significant and valuable insights for further formulation work, with a focus on optimizing drug candidates regarding future clinical studies involving RNX-022 and RNX-023. Moreover, the Company’s drug candidates for the treatment of chronic leg ulcers (RNX-022, RNX-023) and Crohn’s Disease (RNX-041) remain of great clinical and commercial interest. These programs will continue to advance through strategic clinical and industrial collaborations.
Analyst Group’s view of the new execution strategy and prioritization of clinical areas
“Analyst Group views the new execution strategy and prioritization of clinical areas as a positive step, enabling the Company to allocate resources to drug candidates with the shortest routes to market. Prioritizing these programs, supported by robust clinical data and patent protection, enhances the regulatory and commercial potential, which may accelerate the path to licensing agreements and cash flows.
The colorectal cancer market, which PEG addresses through RNX-051, was valued at USD 19bn in 2022 and is estimated to witness a 4.0% CAGR from 2022 to 2030, reaching USD 26bn by 2030.1 In the year 2020, approximately 12.7% of new cancer diagnoses and 12.4% of cancer-related deaths were attributed to colorectal cancer in EU-27 countries – this positioning marks it as the second most prevalent cancer, following breast cancer, and the second leading cause of cancer-related mortality, after lung cancer.2 Hence, the need of new innovative treatment methods in regards to colorectal cancer can be included in effective cancer treatment is more urgent than ever.
Looking at the market that RNX-011 targets, secondary peritonitis poses an increasing challenge and burden for individuals as well as the healthcare system, constituting 1% of urgent hospital admissions and ranking as the second most common cause of sepsis (blood poisoning).3 The global peritonitis treatment market is expected to grow at a CAGR of 6.1% from 2020 to 2028, driven by a rising prevalence and increased investments in R&D to develop permanent and adequate treatment options, originating from both public and government sectors.4
Additionally, the need adequate treatment methods for pouchitis speaks in favor of RNX-041, where a heightened preference for effective yet less invasive symptomatic therapeutics and biologics are fueling the market growth. The global inflammatory bowel disease (IBD) market had, according to ReportLinker, an estimated value of USD 21.3b in 2023 and is expected to experience a 4.8% CAGR during 2023-2027, thereby reaching USD 25.7bn by 2027.5
In summary, Analyst Group believes that focusing on drug candidates with the most favorable near-term outlook and efficiently allocating time and capital is the right approach. With a strengthened balance sheet following the recent directed share issue of.. , a more streamlined focus, and several potential triggers anticipated in early 2025, PEG is well positioned to make significant progress toward licensing agreements in the coming year.”
1. https://www.databridgemarketresearch.com/reports/global-colorectal-cancer-treatment-market
2. https://ecis.jrc.ec.europa.eu/sites/default/files/2023-12/Colorectal_cancer_en-Mar_2021.pdf
3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6889898/?report=printable
4. https://www.databridgemarketresearch.com/reports/global-peritonitis-treatment-market
5. https://www.reportlinker.com/p06317616/Inflammatory-Bowel-Disease-Treatment-Global-Market-Report.html?utm_source=GNW
Comment on PEG’s Q3 Report for 2024
2024-11-15
Pharma Equity Group (“PEG” or “the Company”) published its Q3 report for 2024 on the 15th of November 2024. The following are key events that we have chosen to highlight in the report:
- Patent Protection in Japan (RNX-051)
- Solid Cost Control
- Receivable from Portinho S.A. – Valued at DKK 58m
- Directed Share Issue Strengthens the Balance Sheet
Obtained Patent Protection in Japan for RNX-051
Following the end of the third quarter, the Company’s subsidiary Reponex obtained patent protection in Japan for the treatment of colorectal cancer with RNX-051, valid until 2039. The granting of a patent covering the Japanese market is a significant milestone for Reponex, which has now obtained patent protection for the RNX-051 treatment method in both Europe and Japan – two of the Company’s primary focus markets. Analyst Group assess that the patent protection is an important milestone, given that the patent not only offers legal protection for the Company’s drug candidate but also strengthens PEG’s position as a valuable asset in negotiations with prospective licensing partners.
Robust Cost Control
During Q3-24, the Company’s operating costs totaled approx. DKK 5.2m (5.7), a decrease of -8% Y-Y and an increase of 5% Q-Q. Breaking down the OPEX more in detail, it’s evident that the R&D costs have decreased by -29% Y-Y and -3% Q-Q, while the administrative costs have witnessed a Y-Y increase of 8%, and a sequential increase of 10% Q-Q. Hence, PEG demonstrates a solid cost control Y-Y, and the increased cost base Q-Q is in line with the Company’s maintained guidance for the full year 2024, with EBT expected to be in the range of DKK -24 to -29m (excl. potential gains/losses related to the Portinho receivable).
Receivable from Portinho S.A.
At the end of Q3-24, the receivable from Portinho S.A. was valued at DKK 58m on the balance sheet, similar to the end of the previous quarter. As commented in previous reports, PEG filed a summons with the Maritime and Commercial High Court against Portinho S.A. for the recovery of the receivable of EUR 9.55m plus interest in Q2-24. Analyst Group has excluded the receivable from PEG’s valuation, considering it as an option. If successfully redeemed, this could play a crucial role in supporting the Company’s financial stability and adding further upside to the Company’s valuation, serving as a trigger ahead.
Directed Share Issue Strengthens the Financial Position
After the end of the third quarter, PEG issued 204,592,776 new shares in a directed issue, with gross cash proceeds of approx. DKK 51.1m, including the conversion of convertible debt of approx. DKK 12.6m. Given that the conversion of convertible debt does not involve an actual cash inflow, DKK 38.5m was received in cash. PEG used DKK 25.8m to reduce financial debt and strengthen the balance sheet, thereby resulting in a net cash proceed of DKK 12.7m following the rights issue. The subscription price was DKK 0.25 per share, which corresponded to a premium of approx. 19% in relation to the closing price of the previous trading day, DKK 0.21 on October 3rd. Through the capital increase, the Company achieves a strengthened and more robust capital structure, including an enhanced capital base.
PEG has shown an operational burn rate of approx. DKK -4.5m (-2.7) during Q3-24, equivalent to DKK -1.5m/month, marking an increase from the previous quarter’s monthly burn rate of DKK -1.3m. The increased burn rate stems from a somewhat higher cost base, a natural step as PEG progresses in development, taking further steps towards lucrative licensing agreements. Although PEG reports a slightly increased burn rate, Analyst Group believes PEG manages the operational cost on a good level. With the reported cash balance at the end of Q3-24 (DKK 3.8m), net cash proceeds of DKK 12.7m and a reduced debt burden following the capital raise in October, PEG’s enhanced balance sheet creates more financial flexibility, a key pillar to pursue potential licensing agreements and continue with clinical development.
In summary, the directed share issue was a crucial milestone for PEG, ensuring the continuation of the promising development of the Company’s drug candidates. It also allows PEG to expedite the transition from early discussions with potential licensing partners to formal commercial agreements, which could significantly drive value in the future. Furthermore, we regard the terms of the share issue as highly favorable, particularly the 19% premium on the subscription price, which demonstrates strong confidence from the investors involved in the capital raise. With a strengthened financial position, solid cost control and patent obtained in a key market, PEG has a robust foundation for further clinical progress for the Company’s strong pipeline of candidates.
We will return with an updated equity research report of PEG.
Comment on Pharma Equity Group’s obtained patent protection in Japan for RNX-051
2024-10-23
Pharma Equity Group (“PEG” or “the Company”) announced on Wednesday, October the 23rd, that the Company’s subsidiary Reponex Pharmaceuticals A/S (“Reponex”) has obtained patent protection in Japan for the treatment of colorectal cancer with RNX-051, valid until 2039.
The granting of a patent covering the Japanese market is a significant milestone for Reponex, which has now obtained patent protection for the RNX-051 treatment method in both Europe and Japan – two of the Company’s primary focus markets. Patent protection is seen as a key value driver and a crucial parameter in the future negotiations on the conditions for a potential license agreement.
Colorectal cancer is a significant public health problem in Japan, with both incidence and prevalence increasing in recent decades. It’s the most common cancer type in Japan, driven by an aging population, lifestyle changes, including changes in dietary habits, such as increased consumption of red meat and processed foods, as well as limited physical activity.
“We are very pleased that the Japan Patent Office has granted a patent for our innovative treatment method covering the Japanese market, which will thus be included as a significant positive parameter in our investment rationale of continued clinical development and our dialogue with future license partners,” says Thomas Kaas Selsø, CEO of PEG.
Internationally, there is increasing scientific recognition of the link between the presence of biofilms and the development of colorectal cancer. The biofilm has a major negative impact on the immune system’s ability to detect and fight cancer cells. To give a deeper context, PEG announced positive final results from the Phase II clinical proof-of-concept trial of the drug candidate RNX-051 in Q2-24. In connection to receiving the positive results, Reponex’s management concluded that its patented medicinal product RNX-051 appears to be highly effective for its intended purpose, and just a single local application drastically reduces tumor-associated biofilm. Additionally, a single local application can even totally eliminate the cancer-promoting Fusobacterium nucleatum in the tumor one week after the treatment.
Analyst Group’s view of the obtained patent
“Analyst Group views the approval of the patent application as a significant milestone in PEG’s IP and out-licensing strategy. The patent not only offers legal protection for the Company’s drug candidate but also strengthens PEG’s position as a valuable asset in negotiations with prospective licensing partners.
The colorectal cancer market was valued at USD 19bn in 2022 and is estimated to witness a 4.0% CAGR from 2022 to 2030, reaching USD 26bn by 2030.1 In the year 2020, approximately 12.7% of new cancer diagnoses and 12.4% of cancer-related deaths were attributed to colorectal cancer in EU-27 countries – this positioning marks it as the second most prevalent cancer, following breast cancer, and the second leading cause of cancer-related mortality, after lung cancer.2 Hence, the need of new innovative treatment methods in regards to colorectal cancer can be included in effective cancer treatment is more urgent than ever.
In summary, the indication area targeted by RNX-051 presents immense potential, offering PEG a substantial market share opportunity. A granted patent not only strengthens PEG’s IP portfolio but also opens doors to strategic partnerships, potential royalty streams, and consequently, profitability.”
Analyst Group’s View of Pharma Equity Group
Pharma Equity Group (“PEG” or “the Company”), through the Company’s subsidiary, Reponex, employs a drug repositioning strategy, which involves finding new uses for active substances used in previous recognized treatments, thus allowing the Company to circumvent phase I trials. PEG has a pipeline of six candidates in Phase II, targeting therapeutic areas such as Peritonitis, Chronic Wounds, IBD, and Colorectal Cancer, where there is currently no adequate treatment. The business strategy involves out-licensing the programs after Phase II to a pharma company capable of bringing the drugs to the market. PEG’s strategy enables a capital-light and highly scalable business model, offering a shorter route to market with equivalent upside potential, yet mitigating the typical risks associated with the pharmaceutical industry.
1https://www.databridgemarketresearch.com/reports/global-colorectal-cancer-treatment-market
2https://ecis.jrc.ec.europa.eu/sites/default/files/2023-12/Colorectal_cancer_en-Mar_2021.pdf
Comment on Pharma Equity Group’s Directed Share Issue to a Premium
2024-10-07
Pharma Equity Group (“PEG” or “the Company”) announced on Friday, October 4th, that the Company’s board of directors has resolved to issue 204,592,776 new shares in a directed issue, with expected gross cash proceeds of approx. DKK 51.1m, including the conversion of convertible debt of approx. DKK 12.6m. As a result, the expected net cash proceeds are approx. DKK 38.5m before issue costs, given that the conversion of convertible debt does not involve an actual cash inflow. The subscription price is DKK 0.25 per share, which corresponds to a premium of approx. 19% in relation to the closing price of DKK 0.21 on October 3rd, and the new shares are subscribed by a limited group of new investors and existing shareholders. The dilutive effect following the issuance of new shares amounts to approx. 17% for existing shareholders. Through the capital increase, the Company achieves a strengthened and more robust capital structure, including an enhanced capital base.
Analyst Group’s View of the Capital Increase
“We view the directed issue as a highly positive sign, as it strengthens PEG’s balance sheet substantially, thereby creating financial flexibility and more room for pursuing the development of the Company’s drug candidates. What stands out in particular is that the issue is being conducted at a 19% premium compared to the closing price on October 3rd, which is uncommon in the current market climate and sends a strong signal, as it indicates robust confidence in PEG’s future by the investors participating in the directed issue.
With gross cash proceeds of approx. DKK 51.1m, of which approx. DKK 12.6m stems from the conversion of convertible debt, PEG not only reduces the debt substantially but also has net cash proceeds of approximately DKK 38.5 million before issue costs, resulting in an enhanced financial position and a stronger balance sheet. Considering PEG’s total debt at the end of Q2-24, which amounted to approx. DKK 43.5m, the Company could potentially, with the gross proceeds of approx. DKK 51.1m, diminish the debt and still have net proceeds of approx. DKK 7.6m, all else being equal. Analyst Group views it as likely that PEG will use part of the proceeds to further strengthen the balance sheet by paying the outstanding debt. However, we also deem it likely that a portion of the net proceeds will be used to support further clinical advancements of the Company’s drug candidates in Phase II, as well as to provide the financial capacity to explore potential licensing agreements.
To summarize, Analyst Group views the capital increase as a vital step in enabling PEG to continue the development of the promising studies of the Company’s drug candidates. Additionally, it enables PEG to accelerate the conversion of initial discussions with potential licensing partners into commercial licensing agreements, which could serve as a substantial value driver going forward. Moreover, we view the terms of the directed share issue as favorable, particularly the 19% premium of the subscription price, which sends strong signals from investors participating in the capital raise. This is especially notable in light of the fact that many other small-cap companies are being forced to offer issues at substantial discounts, often coupled with lower subscription commitments, which results in costly underwriting guarantees.”
Analyst Group’s View of Pharma Equity Group
Pharma Equity Group (“PEG” or “the Company”), through the Company’s subsidiary, Reponex, employs a drug repositioning strategy, which involves finding new uses for active substances used in previous recognized treatments, thus allowing the Company to circumvent phase I trials. PEG has a pipeline of six candidates in Phase II, targeting therapeutic areas such as Peritonitis, Chronic Wounds, IBD, and Colorectal Cancer, where there is currently no adequate treatment. The business strategy involves out-licensing the programs after Phase II to a pharma company capable of bringing the drugs to the market. PEG’s strategy enables a capital-light and highly scalable business model, offering a shorter route to market with equivalent upside potential, yet mitigating the typical risks associated with the pharmaceutical industry.
You can access our latest analysis of Pharma Equity Group here, and also watch a recent interview with the CEO, Thomas Kaas Selsø here.
Comment on PEG’s Q2 Report for 2024
2024-08-16
Pharma Equity Group (“PEG” or “the Company”) published its Q2 report for 2024 on the 16th of August 2024. The following are key events that we have chosen to highlight in the report:
- Positive Final Results from Phase II Clinical PoC Trial (RNX-051)
- Robust Cost Control
- Update Regarding the Receivable from Portinho S.A.
- Evaluating Options for a Capital Increase
Continued Advancement of the Clinical Development
During the quarter, PEG announced that the Company’s subsidiary, Reponex Pharmaceuticals A/S (“Reponex”) has received positive final results from the Phase II clinical proof-of-concept trial of the drug candidate RNX-051, also referred to as the MEFO study. Reponex’s management concludes that its patented medicinal product RNX-051 is highly effective for its intended purpose. Just a single local application drastically reduces tumor-associated biofilm and can even totally eliminate the cancer-promoting Fusobacterium nucleatum in the tumor one week after the treatment. Analyst Group believes that the positive results obtained from the Phase II study are a further demonstration from Reponex that the clinical development is progressing according to plan.
Improved Cost Control Compared to Previous Quarter
During Q2-24, the Company’s operating costs totaled approx. DKK 5m (4.9), an increase of 1% Y-Y and a reduction of -27% Q-Q. Breaking down the OPEX more in detail, it’s evident that the R&D costs have decreased by -27% Y-Y and -28% Q-Q, while the administrative costs have witnessed a Y-Y increase of 30%, but a sequential decrease of -26% Q-Q. Hence, PEG maintains a costbase on par with the same period last year and a substantial improvement compared to the previous quarter (Q1-24). As mentioned in the report, PEG maintains the Company’s guidance for the full year 2024, with EBT expected to be in the range of DKK -24 to -29m (excl. potential gains/losses related to the Portinho receivable).
Legal Actions to Redeem the Receivable from Portinho S.A.
At the end of Q2-24, the receivable from Portinho S.A. was valued at DKK 58m on the balance sheet, similar to the end of the previous quarter. As of April 15th, PEG filed a summons with the Maritime and Commercial High Court against Portinho S.A. for the recovery of the receivable of EUR 9.55m plus interest, equivalent to EUR 10.8m or DKK 80.5m. The receivable amount, as per the end of Q2-24, including agreed interest, is EUR 11.0m corresponding to DKK 82.1m. Interest rate is agreed to 2% per quarter and amounts to DKK 3.2m for H1-24, which has not recognized as income in the report as it is considered appropriate to defer income recognition of interest until interest has been paid. Analyst Group has not factored in the receivable in the valuation of PEG and views this as an option which, if redeemed successfully, could be of significant importance to sustaining the Company financially and providing additional upside to the valuation.
Exploring Options Regarding Capital Increase
The Company’s cash balance at the end of June 2024 amounted to approx. DKK 0.9m, a decrease of DKK -1.3m compared to approx. DKK 2.2m at the end of Q1-24. PEG has shown an operational burn rate of approx. DKK -3.9m during Q2-24, equivalent to DKK -1.3m/month, marking a substantial decrease from the previous quarter’s monthly burn rate of DKK -2.6m. However, it’s worth mentioning that the working capital cycle has a fluctuating pattern, and the effect often smooths out over the year. Regardless of that, Analyst Group see it as important to keep the burn rate as low as possible until the Company has secured additional financing, which PEG managed to do during Q2-24.
PEG announced during Q2-24 that the Company is exploring the possibilities regarding a directed capital increase at market price. Since then, no directed capital increase has taken place, but the Company has issued additional convertible loans which allow PEG to borrow DKK 2m. The net debt position amounted to DKK 42.7m at the end of June, compared to a net debt of DKK 36.6m at the end of Q1-24, marking an increase of DKK 6.1m in absolute terms. PEG has an unused credit facility amounting to approx. DKK 5m, which could further strengthen the Company’s liquidity position.
With the latest reported cash position (DKK 0.9) and unused credit facility (DKK 5m), Analyst Group deem it likely that PEG will pursue some form of capital increase in the coming quarters to further strengthen the liquidity position, which is in line with the communication from the Company.
In summary, it is of importance to see further positive progress in clinical development, with the positive final results from the Phase II proof-of-concept trial for the drug candidate RNX-051 serving as a testament to this. Examining the operational cost base, it’s evident that the Company has taken important measures in terms of cost control, which is critical until additional financing is secured. Analyst Group believes that the legal actions taken to redeem the receivable from Portinho S.A. are crucial to increasing the likelihood of recovering the cash, although in an ideal scenario, these measures would have been avoided. If the receivable is successfully recovered, it could not only be of significant importance in sustaining PEG financially, but also serve as a trigger for the share price going forward. Going forward, Analyst Group believes it will be crucial to secure additional capital to maintain the financial flexibility needed, particularly for further clinical development and exploring potential licensing agreements. Additionally, further clinical progress for the pipeline candidates is an important aspect to monitor, as positive data will be a key asset in discussions with potential licensing partners.
We will return with an updated equity research report of PEG.
Comment on Pharma Equity Group’s Positive Final Results From the Phase II Trial of the Drug Candidate RNX-051
2024-04-05
Pharma Equity Group (“PEG” or “the Company”) announced on Friday, April 5th, that the Company’s subsidiary Reponex Pharmaceuticals A/S (“Reponex”) has received positive final results from the Phase II clinical proof-of-concept trial of the drug candidate RNX-051.
The Phase II trial, also referred to as the MEFO trial, concerns the treatment of patients with right-sided colon cancer and right-sided colon polyps/adenomas (precursors of cancer) with the Company’s drug candidate RNX-051. The trial consisted of two arms: the first in patients with adenomas (the “adenoma arm”) and second in patients with cancers in the right side of the bowel (the “cancer arm”). In the adenoma arm, the main goal of the study, to demonstrate an impact on the bacterial biomass, was reached, with a massive reduction in the biofilm of the bowel lining (more than 30-fold reduction). In the cancer arm, for patients with a high content of bacterial biofilm, there was a statistically significant reduction of biofilm in the tumor periphery.
Reponex’s management concludes that its patented medicinal product RNX-051 appears to be highly effective for its intended purpose. Just a single local application drastically reduces tumor-associated biofilm and can even totally eliminate the cancer-promoting Fusobacterium nucleatum in the tumor one week after the treatment.
Analyst Group’s view
“The positive results obtained from the Phase II study are a further demonstration from Reponex that the clinical development is progressing according to plan. The recently strengthened cash position following the convertible loans provides the Company with additional room to maneuver. Coupled with clinical progression, it de-risks the investment case and reinforces PEG’s negotiation power in discussions with potential licensing partners.
During 2020, approximately 12.7% of new cancer diagnoses and 12.4% of cancer-related deaths were attributed to colorectal cancer in EU-27 countries, making it the second most prevalent cancer, following breast cancer, and the second leading cause of cancer-related mortality after lung cancer.1 Hence, the demand for an effective and localized treatment solution is critical.
Analyst Group estimates that the potential royalties from RNX-051 will constitute a significant portion of the total pre-risk-adjusted royalties, making it a key candidate for future potential cash flow streams. The figure below illustrates Analyst Group’s estimates for colorectal cancer (RNX-051).”
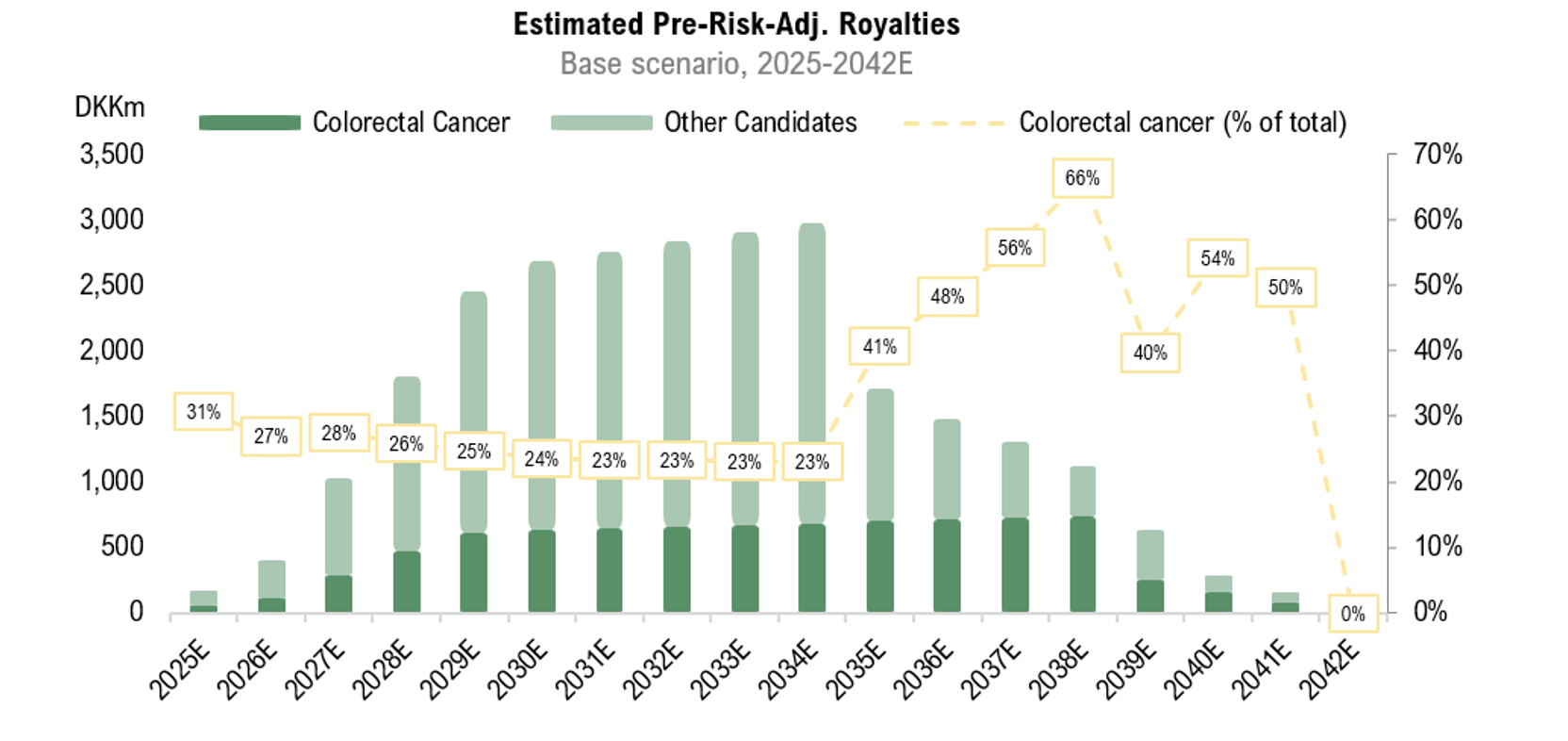
Analyst Group’s View of Pharma Equity Group:
Pharma Equity Group (“PEG” or “the Company”), through the Company’s subsidiary, Reponex, employs a drug repositioning strategy, which involves finding new uses for active substances used in previous recognized treatments, thus allowing the Company to circumvent phase I trials. PEG has a pipeline of six candidates in Phase II, targeting therapeutic areas such as Peritonitis, Chronic Wounds, IBD, and Colorectal Cancer, where there is currently no adequate treatment. The business strategy involves out-licensing the programs after Phase II to a pharma company capable of bringing the drugs to the market. PEG’s strategy enables a capital-light and highly scalable business model, offering a shorter route to market with equivalent upside potential, yet mitigating the typical risks associated with the pharmaceutical industry. Based on an rNPV-model, a potential present value per share of DKK 1.4 is derived in a Base scenario.
You can access our initial analysis of Pharma Equity Group here, and also watch a recent interview with the CEO, Thomas Kaas Selsø here.
1https://ecis.jrc.ec.europa.eu/pdf/factsheets/Colorectal_cancer_en-Mar_2021.pdf
Aug
Interview with Pharma Equity Group’s CEO Thomas Kaas Selsø
Share price
N/A
Valuation Range
2025-03-24
Bear
0.2 DKKBase
0.8 DKKBull
1.6 DKKDevelopment
Principal shareholder
2024-12-31


Comment on PEG’s Year-End Report for 2024
2025-03-20
Pharma Equity Group (“PEG” or “the Company”) published its Year-End report for 2024 on the 20th of March 2025.
The following are key events that we have chosen to highlight in the report:
New Execution Strategy Set to Accelerate the Road to Licensing Agreements
During the fourth quarter, the board approved a new execution strategy and a refined prioritization of clinical focus areas within the Company’s subsidiary, Reponex Pharmaceuticals A/S (“Reponex”). As part of PEG’s evaluation of Reponex’s clinical pipeline, several key commercial factors were assessed, including medical need, patient recruitment feasibility, regulatory pathways, probability of success, and resource allocation in terms of both human and financial capital. Based on these considerations, Reponex has prioritized the following development programs, which have demonstrated clinically relevant data and hold patent protection in key geographical markets, reinforcing the Company’s strategic positioning in the sector:
Recent Clinical Advancements
At the beginning of Q1-25, PEG submitted trial applications for RNX-011 to the authorities, aiming to initiate a Phase 2 clinical trial with two distinct treatment arms: a placebo group and a treatment group receiving RNX-011. The trial is set to enroll 32 patients, evenly distributed between the two arms. Furthermore, the Company expects to submit trial applications for RNX-051 in late Q1-25 or early Q2-25. This trial, conducted in collaboration with SUH Køge and its international research network, will be a larger, placebo-controlled Phase 2 study involving approx. 400 patients, focusing on individuals with colon adenomas. Regarding RNX-041, the drug candidate is actively included in Part 2 of the ongoing Phase 2 proof-of-concept clinical study for the treatment of pouchitis. The Company is actively pursuing strategic partnerships to support larger clinical trials, which could further accelerate the clinical development process.
While PEG has adopted a new execution strategy and refined the Company’s clinical priorities, PEG’s drug candidates for chronic leg ulcers (RNX-022, RNX-023) and Crohn’s Disease (RNX-041) remain of significant clinical and commercial interest. These programs will continue through strategic clinical and industrial collaborations.
Analyst Group assesses that the streamlined focus area strengthens PEG’s ability to allocate resources efficiently, prioritize high-potential drug candidates, and accelerate clinical progress. By concentrating efforts on targeted therapeutic areas, PEG enhances the Company’s prospects for securing strategic partnerships, optimizing trial outcomes, and ultimately increasing the likelihood of a successful commercialization through lucrative licensing agreements.
Guidance for FY 2025 – Ongoing Licensing Discussions are Expected to Convert to Revenue
The Company is currently in dialogue with potential licensing partners, and PEG anticipates entering license agreements by the end of Q3-25 and Q4-25. This is reflected in the estimated FY 2025 revenue of DKK 11m, primarily driven by forecasted upfront payments. Moreover, PEG projects a significant reduction in the cost base for 2025 compared to both 2023 and 2024, with cost-cutting measures and the conversion of fixed to variable costs playing a key role in lowering capital requirements. Overall, the Company expects a pre-tax loss (EBT) of DKK 4–7m, including revenue from licensing agreements. The total expected cash outflow for 2025 is approx. DKK 14.5m, representing a significant improvement from 2024, when operating cash flow (OCF) stood at DKK -22.8m. Analyst Group believes that the ongoing licensing discussions serve as key triggers for 2025, as PEG takes critical steps toward securing lucrative agreements and generating cash flow.
Solid Cost Control Supports Accelerated Development
During Q4-24, the Company’s operating costs totaled approx. DKK 4.2m (6.5), a decrease of 36% Y-Y and 19% Q-Q, a testament to the cost-cutting measures bearing fruit. Breaking down the OPEX more in detail during Q4-24, the R&D costs have decreased by 3% Y-Y and increased by 57% Q-Q, as the Company continues to progress with the development of the pipeline candidates. Furthermore, the administrative costs have witnessed a Y-Y and Q-Q decrease of 61% and 59%, respectively, a solid indication of robust cost control. Overall, the EBT for 2024 came in at DKK -26.2m, in line with the Company’s guidance for the full year (loss of DKK 24-29m).
It is worth noting that the current management has assessed that the capitalized development costs related to projects and patents did not meet the criteria set by IAS 38. Consequently, the Company has reduced Development Projects (intangible assets) from approx. DKK 13.6m to zero, a technical adjustment that lowers opening equity but does not impact financial results or taxes for 2023–2024.
Receivable from Portinho S.A.
At the end of Q4-24, the receivable from Portinho S.A. remained valued at DKK 58m on the balance sheet, consistent with the previous quarter. As noted in earlier reports, PEG filed a summons with the Maritime and Commercial High Court in Q2-24 against Portinho S.A. to recover approx. EUR 9.6m plus interest. PEG states in the Q4 report that a decision in this case is not expected in 2025. Additionally, arbitration proceedings against Interpatium, the real estate developer on Madeira Island, are ongoing before the Danish Institute of Arbitration (DIA) concerning the sale of shares in Portinho.
Enhanced Financial Flexibility Through Directed Share Issue
At the beginning of the fourth quarter, PEG successfully strengthened the Company’s financial position through a directed share issue, generating gross proceeds of approx. DKK 51.1m. This includes the conversion of convertible debt amounting to DKK 12.6m, which, as a non-cash transaction, resulted in a net cash inflow of DKK 38.5m. Of this amount, the Company allocated DKK 25.8m to reduce financial debt, further reinforcing the Company’s balance sheet, leaving a net cash proceed of DKK 12.7m post-debt reduction.
Notably, the shares were issued at a price of DKK 0.25 per share, representing a 19% premium to the closing price on October 3rd, a strong indication of investor confidence in PEG’s future prospects. The capital injection not only strengthens PEG’s financial resilience but also enhances the Company’s strategic flexibility, positioning the Company to actively pursue potential licensing agreements and advance the clinical development pipeline.
In early 2025, PEG strengthened the Company’s financial flexibility further through loans and loan commitments totaling approx. DKK 13m. Given the Company’s expected burn rate, this provides a runway exceeding 12 months. The financial headroom is expected to improve further throughout 2025 via convertible loans or similar financing, with ongoing discussions underway with both existing and new investors for short- and long-term funding.
PEG has shown an operational burn rate of approx. DKK -6.7m (-3.8) during Q4-24, equivalent to DKK -2.2m/month, marking an increase from the previous quarter’s monthly burn rate of DKK -1.5m. The increase is driven by changes in working capital and higher interest expenses. With a more streamlined strategy, an improved financial position following the directed share issue, a strong focus on efficiency measures, and expected licensing revenues in 2025, Analyst Group believes PEG is well-positioned to execute the Company’s strategy and advance toward licensing agreements.
Executive Management and Organizational Changes
Following the end of the quarter, the Company announced the appointment of Christian Henrik Tange as the new CEO of PEG, effective April 1st, 2025. Tange brings over 25 years of experience in financial transformation and transactions, including IPOs, equity and debt financing, and M&As across both listed and private companies in Europe and the US. With an extensive network of Nordic, European, American, and Chinese investors, Tange has successfully raised over DKK 500 million for various companies. He has held key positions in international firms and possesses deep expertise in making companies attractive to investors. Among his previous roles, he served as CFO and Investment Manager at Karolinska Development, a Nasdaq Stockholm-listed investment company. Most recently, he was CEO of Capiital, where he specialized in refining corporate strategies and operations, as well as providing tailored advisory, funding, and M&A services.
The current CEO, Thomas Kaas Selsø, will step down from his position on March 31, 2025, to focus on his consulting business. He will continue to support PEG as a consultant, specializing in accounting, finance, and reporting.
In conjunction with these changes, Sebastian Bo Jakobsen has been appointed CEO of PEG’s subsidiary, Reponex. Jakobsen, who holds a master’s degree in cognitive science from Aarhus University, has been with Reponex as Manager of Scientific Development since September 2022. This appointment aims to provide a focused approach to Reponex’s clinical development activities and to intensify efforts in establishing strategic collaborations with potential licensing partners.
Analyst Group assesses that both Christian and Sebastian appear to be strong candidates for their respective roles, possessing qualifications well-suited to the Company’s strategic objectives. Christian’s extensive background in financial transformation and transactions, combined with his broad investor network and prior experience in the pharma industry, represents key strengths in driving PEG’s next phase of financing and, ultimately, licensing agreements for the Company’s pipeline candidates. Having been with Reponex since Q3-22, Sebastian has developed a deep understanding of the current drug candidates, a crucial asset in successfully executing the new strategy and advancing clinical development.
In summary, PEG enters 2025 with ongoing discussions with potential licensing partners, a key driver for unlocking the Company’s hidden value. Simultaneously, PEG has strengthened the Company’s financial position following a directed share issue executed at a 19% premium, alongside a refined execution strategy focused on the most commercially promising market opportunities. Pipeline candidates RNX-051, RNX-011, and RNX-041 constitute the core priorities, supported by a more cost-efficient approach and expected revenues toward the end of 2025 stemming from licensing agreements, which Analyst Group believes will serve as key triggers that could further accelerate value realization in 2025.
With a newly appointed executive management team bringing expertise in securing funding, driving strategic initiatives, and accelerating clinical advancements, PEG is well-positioned to advance the Company’s pipeline and secure licensing agreements. Building on the foundation established under the previous leadership, the strengthened management structure enables PEG to transition more swiftly from early-stage discussions with potential licensing partners to formalized commercial agreements, paving the way for significant long-term value creation.
We will return with an updated equity research report of PEG.